Imagine a leading biotech company that specializes in developing vaccines, biopharmaceuticals, and revolutionary cell & gene therapies, they wanted to work smarter, not harder. That's where Tulip Interfaces and FrontWell Solutions came in - a tech platform and an implementation partner that could make their logistics and manufacturing processes smoother and meet strict quality standards (GxP).
But there was a twist. They wanted to roll out Tulip even before they received their first batch of materials – a challenging goal that required a mix of careful planning and quick adaptability. They weren't just looking for efficiency; they wanted real-time updates on their inventory and a better way to track their supply chain.
In addition, they had warehouses both inside and outside the company, and all these spaces needed to work together seamlessly. Plus, Tulip had to communicate with other important systems they used, like ERP.
So, we split the project into two parts. Phase 1 focused on creating 9 apps to supercharge their logistics, and Phase 2 took on 6 more apps aimed at making manufacturing smoother.
In a nutshell, this was about an innovative company teaming up with Tulip and FrontWell to make things work better, faster, and smarter – from warehouse moves to crafting innovative treatments.
Customer Background
A biotech CRDMO offering research,
development and manufacturing of vaccines, biopharmaceuticals, and cell &
gene therapies was interested in leveraging Tulip to increase efficiency in
logistics and manufacturing and meet strict GxP requirements. The project was
part of a greenfield implementation, with the goal of going live with Tulip
prior to receiving the first batch of materials, which required a combination
of “right-the-first time” and agile methodologies. Other drivers for digitizing
intralogistics processes were real-time visibility of inventory and enhanced
supply chain traceability.
Complexities specific to the customer included external and internal warehouses that needed to be connected for material movement between intralogistics and material cold chain requirements, and integrations with dependent systems (e.g. ERP).
Scope
This was a greenfield project with an
intended go-live prior to receiving first materials. It was approached in two
phases: Phase 1 included development of 9 applications focused on logistics and
phase 2 included development of 6 applications focused on manufacturing.
Project completion was defined as delivery of all the required validation
documentation.
|
Phase 1 Applications |
Phase 2 Applications |
Validation Documentation |
|
·
Goods Receipt |
·
Adhoc Consumption |
·
Validation Plan |
Project Outcomes
FWS suggested an agile and iterative two-phased implementation
approach to Tulip application development to prioritize delivering working
software incremental and quickly allowing value to be realized sooner while
adapting to changing requirements and feedback from the customer and
maintaining strict adherence to Good Manufacturing Practices (GMP):
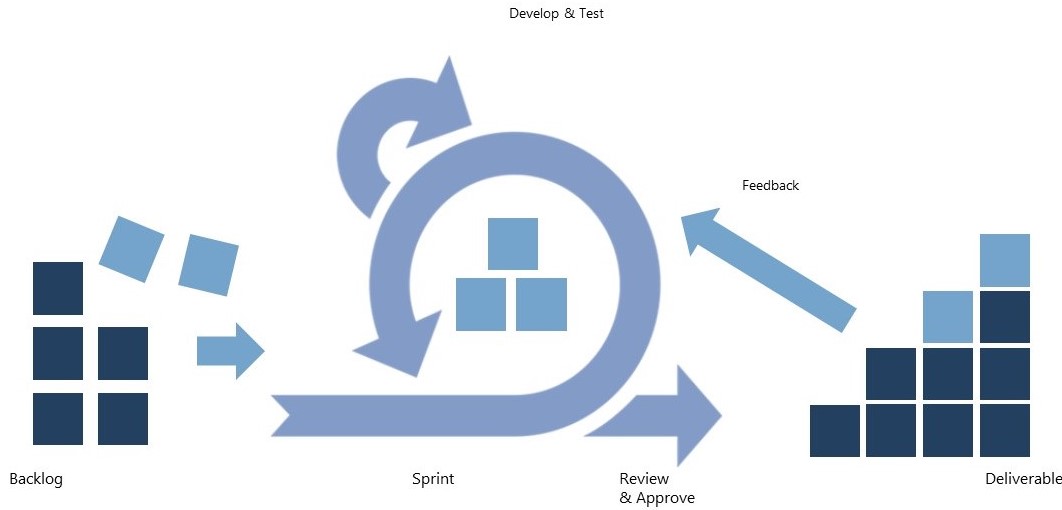
- Workshops to define the Scope: FWS and the customer jointly defined the scope of the project, including the objectives, functional requirements, and regulatory requirements.
- Creation of Product Backlog: FWS created a product backlog outlining the features and functionality required for the applications and prioritized the backlog items based on their regulatory impact and business value.
- Plan Sprints: Sprints were planned that are short and focused, on average 1-4 weeks in length. During each sprint, the team worked on a set of backlog items, delivering a working version of the application at the end of each sprint.
- Develop and Test: All apps were developed and tested in accordance with GMP principles, including testing for functionality, performance, and security.
- Document: Documentation of the app development process and any deviations from the original plan was done by FWS in parallel to the execution.
- Review and Approve: Regular reviews of the app were conducted with all customer stakeholders from production, logistics and QC, to obtain approval from the necessary parties before moving on to the next sprint.
- Deploy and Monitor: FWS deployed the app in a controlled environment and monitored its performance. We used data analytics tools to collect and analyze data from the app to identify any issues or areas for improvement.
- Continuous Improvement: FWS continued to improve the app over time, based on feedback from stakeholders and ongoing monitoring of its performance. Through an iterative approach to app development, making changes and improvements based on user feedback and data analysis was straight-forward.
- The same process was applied to extensions or further rollouts of
the app to other sites.
Leveraging Tulip's no-code UI editor to develop the applications and a CSA validation approach focused on high-risk areas, FWS was able to deliver the entire project in less than 5 months, well within the customer's desired timeline. As Tulip is a cloud platform, this was all accomplished with no server, hardware, or infrastructure requirements.
With FWS developing the applications and all required validation documentation, the customer was only responsible for PQ execution utilizing the provided test plan.
Another important project success was the limited number of required resources. The FWS team consisted of a project manager, solution architect, application developer and validation expert. On the customer side a project lead and SMEs from manufacturing, logistics, validation and IT were involved dedicating at most 5-7 hours of time per week at the height of the project.
After successful completion of this project, an extension of Tulip into the customer’s QC laboratories is planned for 2024.
"Thecollaboration
with FrontWell to implement Tuliphas been a great experience for our team. We’re
engaged in biotech development manufacturing services, so we aimed to optimize
our logistics and manufacturing operations while maintaining GMP compliance.
FrontWell skillfully designed and configured Tulip applications that precisely met
our unique requirements, and we aregenuinely gratefulfor their contribution!
- Head
of Manufacturing, CRDMO
Case Study: Building a digital logistics and manufacturing solution on Tulip for a biotech CRDMO